 Tumor cells, however, can sometimes outwit the immune system by displaying surface proteins that bind with T-cell checkpoint proteins to cause suppression of T-cell activity. In some cases, interaction of these tumor surface proteins with T-cells even causes the T-cells. In recent years, scientists have been trying to develop “checkpoint inhibitor” drugs which will counteract these suppressive checkpoint interactions in order to re-activate the body’s immune response to tumor cells. One of these drugs is U.S. FDA approved to treat metastatic melanoma; others are available or under development to treat other malignancies.
Tumor cells, however, can sometimes outwit the immune system by displaying surface proteins that bind with T-cell checkpoint proteins to cause suppression of T-cell activity. In some cases, interaction of these tumor surface proteins with T-cells even causes the T-cells. In recent years, scientists have been trying to develop “checkpoint inhibitor” drugs which will counteract these suppressive checkpoint interactions in order to re-activate the body’s immune response to tumor cells. One of these drugs is U.S. FDA approved to treat metastatic melanoma; others are available or under development to treat other malignancies. Despite these advances, however, it remains difficult to determine which cancer patients are likely candidates for this type of therapy and which drugs have the most potential. Developing a method to address these challenges would be instrumental in determining the safest, most effective drugs for cancer patients while saving time and money. In order for such a method to be practical for clinical use, it should be able to achieve rapid testing of large numbers of potential immunotherapy drugs against live tumor cells for accurate, easily analyzable data.
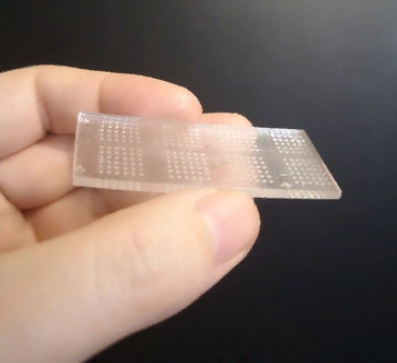
A collaborative team from the Terasaki Institute for Biomedical Innovation (TIBI) has successfully designed and tested such a system. They began by culturing spherical aggregates of breast cancer cells in a custom-fabricated, 3D printed, transparent chip with conical microwells. These microwells were designed for optimum growth and stability of the cellular spheres. Tests performed on the microwells’ cellular spheres confirmed the cells’ viability and their production of T-cell de-activating surface proteins.
“The features of our microwell-based chip is the key to our successful development of an immunoactive tissue model,” said Wujin Sun, Ph.D., from the Terasaki Institute’s team. “The chip’s transparency allows for direct microscopic observation. And its design allows for high-volume testing, which lends itself well to the rapid screening of immunotherapeutic drugs.”
In order to test the effectiveness of checkpoint inhibitor drugs in activating T-cells’ anti-tumor response, the team next considered how a T-cell normally behaves during activation. When a T-cell is stimulated to attack cellular invaders, it secretes proteins called cytokines, which mobilize other immune cells to the invasion site and stimulates the cells to multiply and destroy the invaders. Measurement of these cytokines can therefore indicate the level of a T-cell’s activation.
The team then created an efficient, automated system to measure cytokine levels using their microwell chip. Experiments with this system were performed using anti-checkpoint protein drugs; the results showed that upon incubation of the breast cancer cells with the T-cells, cytokine production was increased by the use of the drugs, demonstrating their effectiveness in activating the T-cells.
Another way the team used their breast cancer chip was to assess the breast cancer cells’ effect on stimulated T-cells The T-cells were fluorescently labeled and added to the breast cancer cells in the microwells; the chip’s transparency allowed direct observation of their cellular interaction using fluorescent microscopy. These breast cancer cells normally cause rupture of the T-cells, but experiments conducted with checkpoint inhibitor drugs showed that the drugs increased T-cell viability in the cultures, visually demonstrating how they can counter the effects T-cell rupture by tumor cell interaction.
The breast cancer chip was also used for the direct observation of how the T-cells infiltrated the breast cancer cellular spheres; this type of infiltration is a measure of a T-cell’s anti-tumor activity and viability After labeling each group of cells with separate dyes and mixing them in the chip’s microwells, T-cell infiltration could be directly visualized using high resolution fluorescence microscopy. Experiments conducted with checkpoin-inhibitor drugs indicated that there were increased numbers of T-cells and deeper penetration into the breast cancer cells in the presence of the drugs.
In summary, the TIBI researchers were able to design robust and efficient methods for characterizing the interaction between tumor and immune cells and for rapid, high-volume and clinically-relevant ways to screen immunotherapeutic drugs against tumor cells. The microwell chipand its related apparatus can also be used to include other types of tumor cells and individual patient cells for optimizing patient response and for screening and developing additional anti-cancer drugs.
“Bringing ways to optimize clinical decisions and personalized medicine for patients is a top goal at our institute,” said Ali Khademhosseini, Ph.D., director and CEO of the Terasaki. “This work is a significant step towards achieving that goal in the realm of cancer immunotherapy.”
terasaki.org


