These consisted of a myostatin inhibitor to protect against loss of muscle mass with age, and a telomerase inducer to battle stem cell depletion responsible for diverse
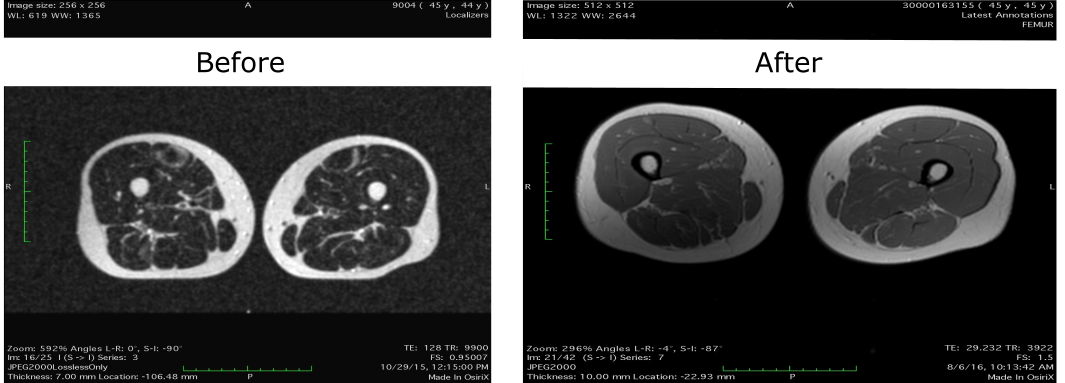
MRI images of subject’s thighs. The left image is before treatment, the right after treatment. A growth in overall mass can be observed in the right sample with a visible reduction in intramuscular triglyceride content.
Further examination of Parrish’s data prior to the therapy and following the therapy has revealed additional positive changes. MRI scans taken before and after depict a slight increase in muscle size in conjunction with a noticeable reduction in muscle fat content. An overaccumulation of intramuscular fat, also known as ‘marbling’, is associated with increased insulin resistance (1), and as such an appropriate reduction may be linked to beneficial metabolic changes in addition to improved musculature. The aforementioned patient’s total body weight has not decreased during this period, and as such weight loss is not a confounding variable. More importantly, this apparent muscle growth was achieved without any exercise regime. We therefore predict that results may be more striking in more physically active individuals. The muscle growth achieved
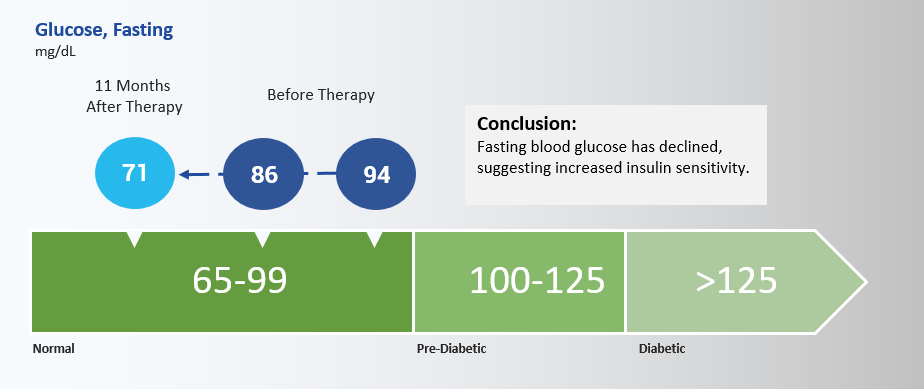
Conclusion: Fasting blood glucose has declined suggesting increased insulin sensitivity.
Bernardes de Jesus et al., 2012 (3), noted that a significant reduction in fasting glucose was apparent in mice following AAV mediated telomerase induction. The subject’s fasting glucose has declined from previous measurements of 94 mg/dL and 86 mg/dL, to a fasting glucose level of 71 mg/dL by August 2016, as measured by Quest Diagnostics. Repeated testing will confirm the implied increase in insulin sensitivity. Previous research has also indicated that telomerase deficiency impairs glucose metabolism and insulin secretion in telomerase deficient mice (4), which may explain an apparent improvement in metabolic markers.
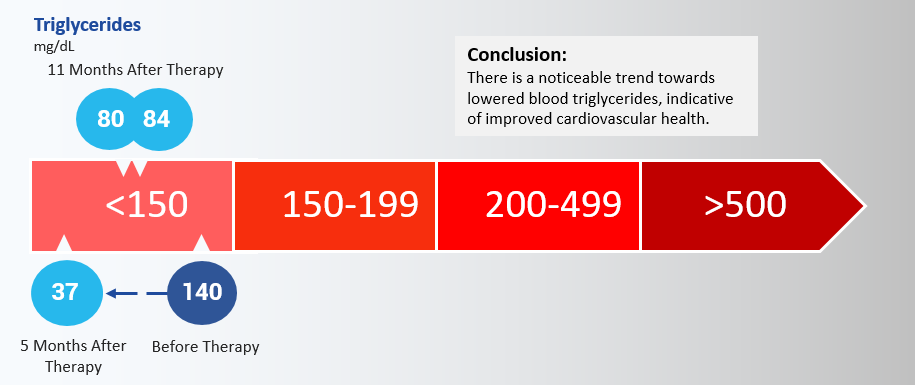
In accordance with an improvement in metabolic health, triglyceride levels also declined from 140 mg/dL in 2015 to 36 mg/dL in February 2016, subsequently rising to 80 and 84 mg/dL in August 2016. While there has been an increase in blood triglyceride content following the February reading, it is still measurably lower than before treatment. Both decreases in fasting glucose and triglycerides can be potentially explained by prior studies of telomerase and myostatin. Raised myostatin mRNA seen in type 2 diabetes patients is associated with impaired insulin sensitivity, raising triglyceride levels and
Conclusion: There is a noticeable trend towards lowered blood triglycerides, indicative of improved cardiovascular health.
In addition to these apparent benefits, blood analysis has revealed further insights into the effects of the complementary therapies.
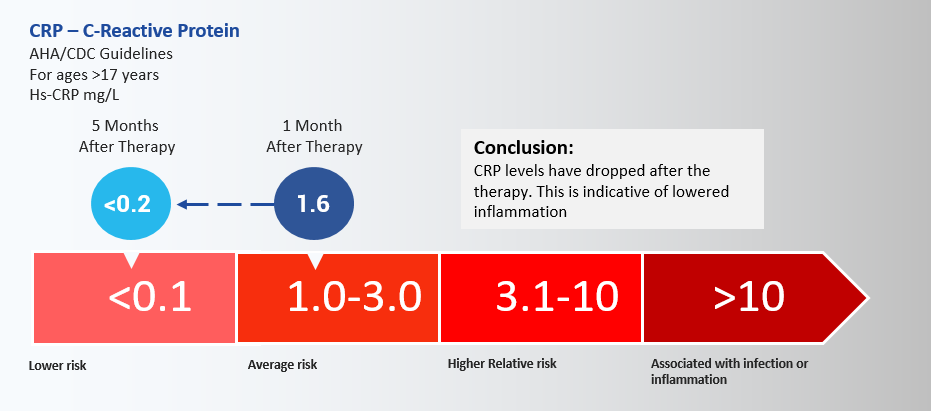
Conclusion: CRP levels have dropped after the therapy. This is indicative of lowered inflammation
No negative effects have been reported. Blood analysis has revealed no detrimental effects thus far, providing tentative evidence of safety in the first human test of BioViva’s dual gene therapy strategy.
«This data first and foremost implies that these therapies are likely safe in humans, which was our primary concern. Additionally, it also reveals potentially beneficial metabolic changes that may conform to previous research findings. These trends may become more significant following further confirmation as we continuously monitor my biomarkers, but we are certainly encouraged by the results we have seen so far.« — Elizabeth Parrish, CEO