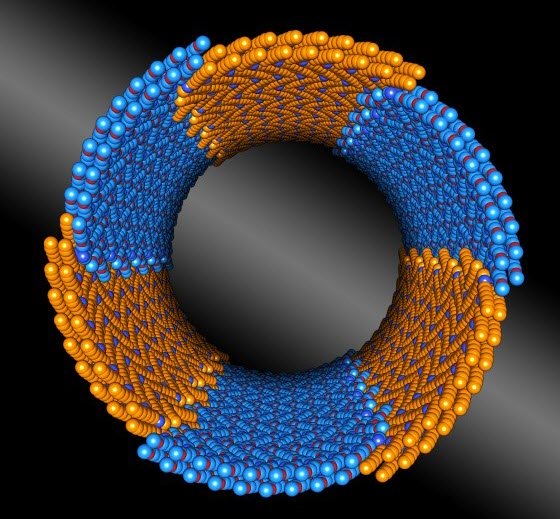
Berkeley Lab scientists discovered a polymer composed of two chemically distinct blocks (shown in orange and blue) that assembles itself into complex nanotube structures with uniform diameters. (credit: Berkeley Lab)
«Creating uniform structures in high yield is a goal in nanotechnology," says Ron Zuckermann, who directs the Biological Nanostructures Facility in Berkeley Lab’s Molecular Foundry, where much of this research was conducted. «For example, if you can control the diameter of nanotubes, and the chemical groups exposed in their interior, then you can control what goes through — which could lead to new filtration and desalination technologies, to name a few examples.»
Accidental discovery of new nanotube design principle
Creating a large quantity of nanostructures with the same trait, such as millions of nanotubes with identical diameters, has been difficult. For the past several years, the Berkeley Lab scientists studied a polymer that is a member of the peptoid family. (Peptoids are rugged synthetic polymers that mimic peptides, which nature uses to form proteins.)
The researchers studied a particular type of peptoid, called a diblock copolypeptoid, because it binds with lithium ions and could be used as a battery electrolyte. In their research, they serendipitously found these compounds form nanotubes in water. They don’t know how exactly, but the important thing with this new research is that it sheds light on their structure, and hints at a new design principle that could be used to precisely build nanotubes and other complex nanostructures.
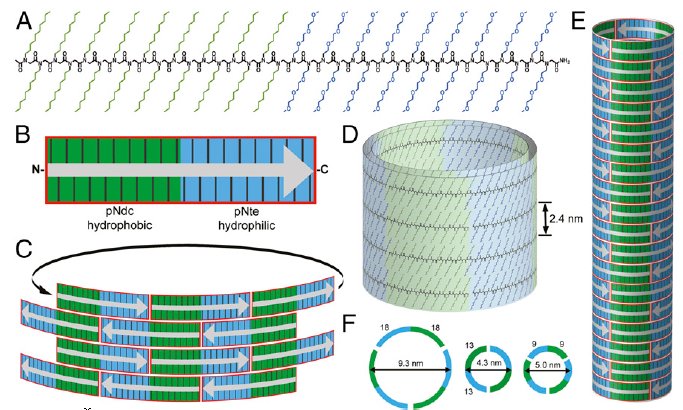
Assembly of peptoid polymer tiles into hollow nanotubes. (A) Chemical structure of a diblock copolypeptoid polymer. (B) Schematic representation of a specific polymer, showing that its tile shape consists of hydrophobic (water-hating) (green) and hydrophilic (water-loving) (blue) domains. Gray arrow indicates the chain orientation. (C) Tiles stack in a brick-like pattern to form stacked horizontal rings (each ring consists of two to three individual peptoid strands), held together by longitudinal side-chain–to–side-chain interactions (D and E). (F) Side view showing the approximate chain configuration and dimensions for each cross-sectional ring. (credit: Jing Suna et al./PNAS)
Remarkably, the nanotubes assemble themselves without the usual
«You wouldn’t expect something as intricate as this could be created without these crutches," says Zuckermann. «But it turns out the chemical interactions that hold the nanotubes together are very simple. What’s special here is that the two peptoid blocks (
The research was reported today (March 28) online in the journal Proceedings of the National Academy of Sciences, supported by the Department of Energy’s Office of Science.
Source: http://www.kurzweilai.net/nature-inspired-precisely-assembled-nanotubes

