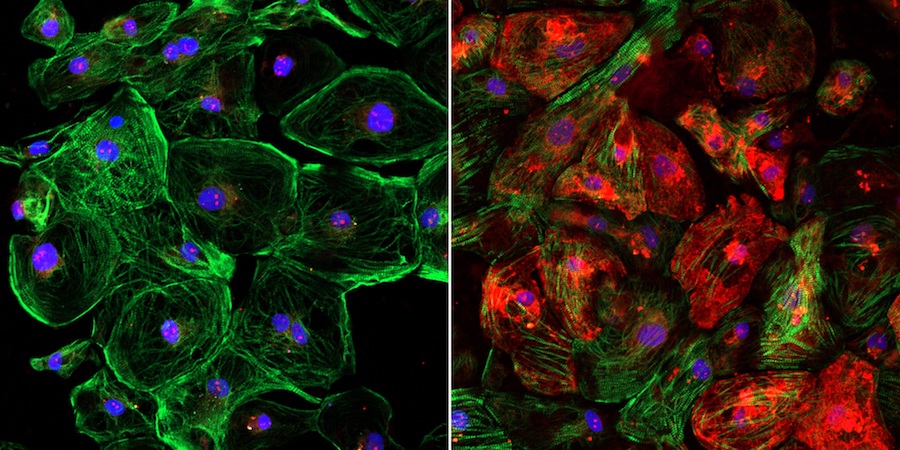
The images illustrate the lack of dystrophin, shown in red, in an unedited human heart muscle cell with DMD (left) and the restoration of dystrophin in a CRISPR-edited human heart muscle cell (right).
DALLAS – March 6, 2019 – Scientists have developed a method to boost the efficiency of CRISPR gene editing in Duchenne muscular dystrophy (DMD), according to a study that could have implications for optimizing gene therapies for other diseases.
The finding stemmed from research at UT Southwestern in which a single-cut gene-editing technique was used on mice and human cells to correct a common mutation that underlies DMD, a fatal disease caused by loss of dystrophin, a protein critical for muscle function.
While testing the technique, scientists discovered that adjusting the dosages of CRISPR’s gene-editing components can significantly improve how much dystrophin is produced by the edited genes. They further found that the optimal ratio of components changed based on which part of the DNA was being edited.
“As we test CRISPR on other defective parts of the dystrophin gene, it may be important to tweak our formulas for optimal results,” said Dr. Eric Olson, who led the study published in Science Advances. “This new insight further facilitates the use of CRISPR as a therapy for Duchenne and perhaps a number of other diseases.”
Unexpected finding

Dr. Eric Olson, Director of UT Southwestern’s Hamon Center for Regenerative Science and Medicine
The gene-editing technique used by the researchers requires two components – an enzyme called Cas9 that cuts DNA and a guide RNA that functions like a molecular GPS device to direct Cas9 to the specific DNA sequence in the genome to be edited.
Dr. Olson’s lab developed a method to edit the defective portion of the dystrophingene by loading Cas9 into an adeno-associated virus (AAV), a harmless virus that is used to deliver the editing components into the cells. The guide RNA is also loaded into an AAV to direct Cas9 to the mutation where it snips the DNA.
The editing process bypasses the mutation and enables muscle fibers to produce dystrophin, with positive results documented in previous studies by Dr. Olson in large mammals, mice, and human cells.
In those studies, the team used a standard 1-to-1 ratio of Cas9 and guide RNA to help restore dystrophin production in muscle to more than 90 percent of normal.
In the new study targeting a different part of the gene, they discovered the 1-to-1 ratio didn’t work as well: Dystrophin production was only restored to 5 percent of normal when delivered into the blood stream.
Through trial and error, the scientists found using a 10-to-1 ratio of guide RNA to Cas9 allowed for optimal editing of that particular segment of the dystrophin gene. Within four weeks of a CRISPR dose, dystrophin production was restored in about 90 percent of muscle and heart fibers in mice with a common DMD mutation.
“This is surprising,” said Dr. Yi-Li Min, the study’s first author. “We had always delivered the guide RNA and Cas9 using two viruses in equal amounts and hadn’t thought to look into changing the ratio.”
Defective exons

Dr. Yi-Li Min, the study's first author
DMD, the most common fatal genetic disease in boys, leads to muscle and heart failure, and premature death by the early 30s. Patients are forced into wheelchairs as their muscles degenerate and eventually onto respirators as their diaphragm muscles weaken. No effective treatment exists, though scientists have known for decades that a defect in any of the 79 exons that comprise the long dystrophingene causes the condition.
Dr. Olson has published multiple studies in which his lab made single cuts at strategic points of DNA to correct mutations. Including his latest study that focused on deletion of exon 44, his lab has created CRISPR techniques that target the five most common defects seen in DMD patients, accounting for about half of cases worldwide. A clinical treatment for exon 44, which is located in one of the most common mutational hotspots on the gene, may benefit about 12 percent of patients.
The techniques have not yet been approved for clinical use, but Dr. Olson’s team took a major step toward that goal last year after publishing a Science study showing that CRISPR edits on exon 51 halted the progression of DMD in dogs.
Within several weeks of the gene editing, the missing protein was restored in muscle tissue throughout the body, including 92 percent correction in the heart and 58 percent in the diaphragm, the main muscle needed for breathing. Scientists have estimated a 15 percent threshold is needed to significantly help patients.
The lab is conducting longer-term studies in dogs to measure whether the dystrophin levels remain stable and to ensure the gene edits do not have adverse side effects.
Dr. Olson hopes the next step beyond dogs is a clinical trial, which would be among several that UT Southwestern’s gene therapy program aims to launch in the coming years to address numerous deadly childhood diseases.
“We have more to do before we can use this clinically,” Dr. Olson said, “but it’s exciting to see how far we’ve come.”


