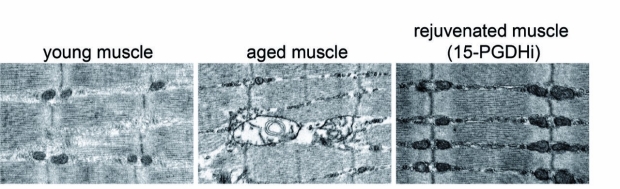
From left, mitochondria in the muscle fibers of young mice, old mice from a control group and old mice treated with a small molecule that inhibits the function of a protein called 15-PGDH.
“The improvement is really quite dramatic” said Helen Blau, PhD, professor of microbiology and immunology. “The old mice are about 15% to 20% stronger after one month of treatment, and their muscle fibers look like young muscle. Considering that humans lose about 10% of muscle strength per decade after about age 50, this is quite remarkable.”
The protein hasn’t previously been implicated in aging. The researchers show that the amount of the protein, called 15-PGDH, is elevated in old muscle and is widely expressed in other old tissues. Experiments they conducted in human tissue raise hopes for a future treatment for the muscle weakness that occurs as people age.
Blau, the Donald E. and Delia B. Baxter Foundation Professor and director of the Baxter Laboratory for Stem Cell Biology, is the senior author of the study, which will be published online Dec. 10 in Science. Senior scientist Adelaida Palla, PhD, is the lead author.
Muscle loss in aging
Muscle loss during aging is known as sarcopenia, and it accounts for billions of dollars of health care expenditures in the United States each year as people lose the ability to care for themselves, experience more falls and become increasingly less mobile. It is due to changes in muscle structure and function: The muscle fibers shrink and the number and function of the cellular powerhouses known as mitochondria dwindle.Blau and her colleagues have long been interested in understanding muscle function after muscle injury and in diseases like Duchenne muscular dystrophy. Previously, they found that a molecule called prostaglandin E2 can activate muscle stem cells that spring into action to repair damaged muscle fibers.
“We wondered whether this same pathway might also be important in aging,” Blau said. “We were surprised to find that PGE2 not only augments the function of stem cells in regeneration, but also acts on mature muscle fibers. It has a potent dual role.”
Prostaglandin E2 levels are regulated by 15-PGDH, which breaks down prostaglandin E2. The researchers used a highly sensitive version of mass spectrometry, a method for differentiating closely related molecules, to determine that compared with young mice, the 15-PGDH levels are elevated in the muscles of older animals, and the levels of prostaglandin E2 are lower.
They found a similar pattern of 15-PGDH expression in human muscle tissues, as those from people in their 70s and early 80s expressed higher levels than those from people in their mid-20s.
“We knew from our previous work that prostaglandin E2 was beneficial for regeneration of young muscles,” Palla said. “But its short half-life makes it difficult to translate into a therapy. When we inhibited 15-PGDH, we observed a systemic elevation of prostaglandin E2 levels leading to a bodywide muscle improvement in aged mice.”
Inhibiting 15-PGDH
The researchers administered a small molecule that blocks the activity of 15-PGDH to the mice daily for one month and assessed the effect of the treatment on the old and young animals.“We found that, in old mice, even just partially inhibiting 15-PGDH restored prostaglandin E2 to physiological levels found in younger mice,” Blau said. “The muscle fibers in these mice grew larger, and were stronger, than before the treatment. The mitochondria were more numerous, and looked and functioned like mitochondria in young muscle.”
Treated animals were also able to run longer on a treadmill than untreated animals.
When Palla and her colleagues performed the reverse experiment — overexpressing 15-PGDH in young mice — the opposite occurred. The animals lost muscle tone and strength, and their muscle fibers shrank and became weaker, like those of old animals.
Finally, the researchers observed the effect of prostaglandin E2 on human myotubes —immature muscle fibers — growing in a lab dish. They found that treating the myotubes with prostaglandin E2 caused them to increase in diameter, and protein synthesis in the myotubes was increased — evidence that prostaglandin E2 worked directly on the muscle cells, not on other cells in the tissue microenvironment.
“It’s clear that this one regulator, 15-PGDH, has a profound effect on muscle function,” Blau said. “We’re hopeful that these findings may lead to new ways to improve human health and impact the quality of life for many people. That’s one of my main goals.”
Blau and Palla are studying more about what controls the levels and activity of 15-PGDH during normal aging, and how it might affect the function of other tissues in the body.
“The mice perform better on a treadmill, but that requires more than just an increase in muscle strength,” Blau said. “Other organ systems are involved — the heart and lungs, for example. It suggests an overall improvement in the function of the whole animal.”
Blau is a member of Stanford Bio-X, Stanford Cardiovascular Institute, Stanford Maternal and Child Health Research Institute, the Stanford Cancer Institute, and the Wu Tsai Neurosciences Institute at Stanford.
Other Stanford co-authors of the research are postdoctoral scholars Meenakshi Ravichandran, PhD, Christian Schürch, MD, PhD, and Yu Xin Wang, PhD; mass spectrometry specialist Ludmila Alexandrova, PhD; former senior scientist Andrew T.V. Ho, PhD; and research assistants Ann Yang, Peggy Kraft and Colin Holbrook.
The research was supported by the Baxter Foundation, the California Institute for Regenerative Medicine, the Li Ka Shing Foundation, the National Institutes of Health (grants 5R01AG02096115, 1RO1AG069858-01, RHG009674A, K99NS120278 and S10OD026962), the Canadian Institutes of Health Research and the Swiss National Science Foundation.
Blau, Palla, Ravichandran and Ho are inventors on patents related to 15-PGDH and licensed to Myoforte Therapeutics, of which Blau is a cofounder. Palla, Blau and Ho receive consulting fees and have equity and stock options from Myoforte Therapeutics.
By KRISTA CONGER
med.stanford.edu


