Authors
This area of investigations is developing by a number of scientists, among them are A. D. de Grey, J. Vijg, R. Holliday et al.
History:
The history of epigenetic researches is linked with studies of evolution and development. For a long time, many scientists did not acknowledged epigenetics at all or even intentionally passed it over in silence.

That occurred mainly due to the fact that knowledge about the nature of epigenetic signals and ways of their realization in the organism was very indistinct. Actually, epigenetics in modern interpretation was developing and promoting by scientists of our country — I.
Example:
Enzygotic twins are known to be clones, i.e. exact genetic copies of each other. In the early childhood, their chromosomes have nearly the same patterns of DNA methylation in the same tissues. Nevertheless, when such twins grow old, they have sharply different patterns of DNA methylation in spite of the genetic identity and the same age.
Description:
Though chromosomes are regularly damaging and the effectiveness of the repair mechanisms declines with age, mutations occur quite rare and slowly accumulate with age.

But the frequency of cancer and other
 When we age, global demethylation of genome takes place, and that activates genes that must normally be «silent». Demethylation is provoked by chromosome damages,
When we age, global demethylation of genome takes place, and that activates genes that must normally be «silent». Demethylation is provoked by chromosome damages,
 Additions and Criticism:
Additions and Criticism:
Life style (including the type of diet) and environment influence substantially the probability of demethylation. For example, deficiency in uptake of vitamins (folic acid, B12) and microelements (zinc, selenium) in old age is one of the reasons of demethylation.
It is not clear as yet why
Publications:
- de Grey, Aubrey DNJ. «Protagonistic pleiotropy: why cancer may be the only pathogenic effect of accumulating nuclear mutations and epimutations in aging." Mechanisms of ageing and development 128.7 (2007): 456–459.
- Vijg, Jan. «The role of DNA damage and repair in aging: new approaches to an old problem." Mechanisms of ageing and development 129.7 (2008): 498–502.
-
Gravina, Silvia, and Jan Vijg. «Epigenetic factors in aging and longevity." Pflügers
Archiv-European Journal of Physiology 459.2 (2010): 247–258. - Holliday, Robin. «Perspectives in aging and epigenetics." Epigenetics of Aging. Springer New York, 2010. 447–455.
 H. Selye pioneered the field of stress research and provided convincing arguments that stress impacted health. From the late 1960s, a large amount of research was undertaken to examine links between stress and disease of all kinds. By the late 1970s, stress had become the medical area of greatest concern. There was also a great amount of laboratory researches into the neuroendocrine, molecular, and immunological bases of stress. By the 1990s, «stress» had become an integral part of modern scientific understanding in all areas of physiology and human functioning, and one of the great metaphors of Western life.
H. Selye pioneered the field of stress research and provided convincing arguments that stress impacted health. From the late 1960s, a large amount of research was undertaken to examine links between stress and disease of all kinds. By the late 1970s, stress had become the medical area of greatest concern. There was also a great amount of laboratory researches into the neuroendocrine, molecular, and immunological bases of stress. By the 1990s, «stress» had become an integral part of modern scientific understanding in all areas of physiology and human functioning, and one of the great metaphors of Western life.

 They can be external (exogenous): increased or decreased environment temperature, fluctuations of oxygen concentrations in the air, injuries, hypodynamia, infections, excess or lack of nutrients, toxins, chemical mutagens, ionizing radiation and untraviolet. They can also be internal (endogenous): the psychological, neurohormonal, oxidative stress, mitochondrial stress and the stress of the endoplasmic reticulum.
They can be external (exogenous): increased or decreased environment temperature, fluctuations of oxygen concentrations in the air, injuries, hypodynamia, infections, excess or lack of nutrients, toxins, chemical mutagens, ionizing radiation and untraviolet. They can also be internal (endogenous): the psychological, neurohormonal, oxidative stress, mitochondrial stress and the stress of the endoplasmic reticulum.
 Recent researches made at the Harvard Medical School have revealed the physiological mechanism binding chronic stress and acute dysfunction of cardiovascular system. It was found that prolonged stress trigger the cascade of reactions which result in excess production of leukocytes — cells of immune system which accumulate in arteries and cause formation of complicated,
Recent researches made at the Harvard Medical School have revealed the physiological mechanism binding chronic stress and acute dysfunction of cardiovascular system. It was found that prolonged stress trigger the cascade of reactions which result in excess production of leukocytes — cells of immune system which accumulate in arteries and cause formation of complicated,  The first recorded observation of an endogenous circadian oscillation was by the French scientist J.-J. d’Ortous de Mairan in 1729 when he studied the movement of the leaves of the plant Mimosa pudica. In 1918,
The first recorded observation of an endogenous circadian oscillation was by the French scientist J.-J. d’Ortous de Mairan in 1729 when he studied the movement of the leaves of the plant Mimosa pudica. In 1918,  Disorders of the internal clock predispose our organism to system inflammation, cancer, cardiovascular diseases, metabolic syndrome and diabetes, neurodegenerative, cognitive and sleep disorders.
Disorders of the internal clock predispose our organism to system inflammation, cancer, cardiovascular diseases, metabolic syndrome and diabetes, neurodegenerative, cognitive and sleep disorders.
 The central (in the brain) and peripheral (in liver, lungs, heart, kidneys, skin) internal body clocks play important role in the regulation of the metabolism, sleep/wake cycles, rhythmicity of the hormones secretion, physical activity, intestinal peristalsis, body temperature, arterial pressure and levels of various metabolites in the blood.
The central (in the brain) and peripheral (in liver, lungs, heart, kidneys, skin) internal body clocks play important role in the regulation of the metabolism, sleep/wake cycles, rhythmicity of the hormones secretion, physical activity, intestinal peristalsis, body temperature, arterial pressure and levels of various metabolites in the blood.
 Tests on laboratory animals have shown that the activity of key genes controlling circadian rhythms comes down as an individual grows older. Thus, mice with mutations reducing activity of the key genes mentioned above live substantially less than normal animals. At the same time, artificial activation of some of those genes in the muscle tissue of mice leads to lifespan extension. Similar results were obtained in researches on fruit flies.
Tests on laboratory animals have shown that the activity of key genes controlling circadian rhythms comes down as an individual grows older. Thus, mice with mutations reducing activity of the key genes mentioned above live substantially less than normal animals. At the same time, artificial activation of some of those genes in the muscle tissue of mice leads to lifespan extension. Similar results were obtained in researches on fruit flies.

 The aging process is characterized by progressive metabolic decline over time, namely by insulin resistance, and physiological declines in growth hormone (GH),
The aging process is characterized by progressive metabolic decline over time, namely by insulin resistance, and physiological declines in growth hormone (GH),  Major impairments include unrestrained hepatic gluconeogenesis, adipose lipogenesis, and defective glycogen synthesis and glucose uptake in skeletal muscle. Abdominal obesity, which is commonly observed with aging, is a major contributor metabolic syndrome.
Major impairments include unrestrained hepatic gluconeogenesis, adipose lipogenesis, and defective glycogen synthesis and glucose uptake in skeletal muscle. Abdominal obesity, which is commonly observed with aging, is a major contributor metabolic syndrome.
 Numerous investigations made by Russian and American researches have shown that activation and suppression of certain genes in hypothalamus (a central neuroendocrine t regulator of the metabolism at the base of the brain) can slow down aging in the whole body.
Numerous investigations made by Russian and American researches have shown that activation and suppression of certain genes in hypothalamus (a central neuroendocrine t regulator of the metabolism at the base of the brain) can slow down aging in the whole body.
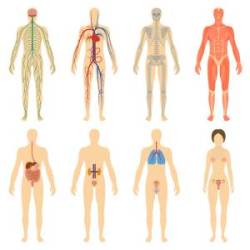 Regulation of metabolism and homeostasis, as well as realization of system functions (respiration, excretion, digestion, blood circulation, immunity) is very essential. Its disturbance causes varied diseases and increases the probability of death. Simultaneously, all the regulatory processes mentioned above are exposed to
Regulation of metabolism and homeostasis, as well as realization of system functions (respiration, excretion, digestion, blood circulation, immunity) is very essential. Its disturbance causes varied diseases and increases the probability of death. Simultaneously, all the regulatory processes mentioned above are exposed to  Physiological regulation of all functions of our organism is under control of the nervous and endocrinous systems, and both of them change substantially during aging. Functioning of some brain regions (e.g. hypothalamus, hypophysis, epiphysis) become worse as an organism ages, and this has negative influence at the functions of peripheral endocrine glands (thyroid body, pancreatic gland, adrenal gland, gonads) and diffuse endocrine system. As the result, the level of many essential hormones goes beyond normal and beyond daily rhythmics, and that increases the risk of tens of diseases.
Physiological regulation of all functions of our organism is under control of the nervous and endocrinous systems, and both of them change substantially during aging. Functioning of some brain regions (e.g. hypothalamus, hypophysis, epiphysis) become worse as an organism ages, and this has negative influence at the functions of peripheral endocrine glands (thyroid body, pancreatic gland, adrenal gland, gonads) and diffuse endocrine system. As the result, the level of many essential hormones goes beyond normal and beyond daily rhythmics, and that increases the risk of tens of diseases.
 At a cell level, aging is also caused by disorders of regulation. A good half of all the proteins acting in the development of
At a cell level, aging is also caused by disorders of regulation. A good half of all the proteins acting in the development of  The fact that genetic instability is the mark of aging is supported by the accelerated aging syndrome. It is caused by congenital mutations in genes controlling DNA repair. As the result of those mutations, young people or even children have signs of aging and even look like elderly people. Mutations induced in genes of DNA repair of mice under experimental conditions also result in accelerated aging.
The fact that genetic instability is the mark of aging is supported by the accelerated aging syndrome. It is caused by congenital mutations in genes controlling DNA repair. As the result of those mutations, young people or even children have signs of aging and even look like elderly people. Mutations induced in genes of DNA repair of mice under experimental conditions also result in accelerated aging.
 The main functions of our body are under control of genes situated in nuclear chromosomes. Every cell has only two copies of each chromosome, and these copies are not identical — they can have different variants of the same gene (alleles). That is why damages of DNA molecules could badly affect cell functions. The surge of mutations that is observed during aging was called genetic instability. An example of genetic instability is telomere shortening. Telomeres are special areas at chromosome ends. They protect chromosomes so cells with defective telomeres cannot divide and even survive, but sometimes such cells begin to divide in an uncontrolled manner and become tumor ones. When we age chromosomes accumulate damage not only in telomere regions but also along the full length. The main reason of that phenomenon is that mechanisms of DNA repair cease to work effectively. Any damage of nucleotides that form genes or breaks in DNA strands cause mutations in aging cell with repair deficiency. The more mutations cell accumulates, the less viable it becomes and the high risk of its transformation into cancer one arises.
The main functions of our body are under control of genes situated in nuclear chromosomes. Every cell has only two copies of each chromosome, and these copies are not identical — they can have different variants of the same gene (alleles). That is why damages of DNA molecules could badly affect cell functions. The surge of mutations that is observed during aging was called genetic instability. An example of genetic instability is telomere shortening. Telomeres are special areas at chromosome ends. They protect chromosomes so cells with defective telomeres cannot divide and even survive, but sometimes such cells begin to divide in an uncontrolled manner and become tumor ones. When we age chromosomes accumulate damage not only in telomere regions but also along the full length. The main reason of that phenomenon is that mechanisms of DNA repair cease to work effectively. Any damage of nucleotides that form genes or breaks in DNA strands cause mutations in aging cell with repair deficiency. The more mutations cell accumulates, the less viable it becomes and the high risk of its transformation into cancer one arises.
 Another reason of genetic instability observed under aging is the activation of mobile genetic elements, also known as «jumping genes» or retrotransposons. Those are
Another reason of genetic instability observed under aging is the activation of mobile genetic elements, also known as «jumping genes» or retrotransposons. Those are  For preventing telomeres shortening in germ and embryonal stem cells the special enzyme — telomerase — is activated in those cells. Telomerase elongates telomeres after every cell division. In the cells of most human tissues and organs, a gene responding for one of the telomerase components is switched off or works poorly. Accessory effect of that
For preventing telomeres shortening in germ and embryonal stem cells the special enzyme — telomerase — is activated in those cells. Telomerase elongates telomeres after every cell division. In the cells of most human tissues and organs, a gene responding for one of the telomerase components is switched off or works poorly. Accessory effect of that  Owing to investigations made by L. Hayflick and
Owing to investigations made by L. Hayflick and 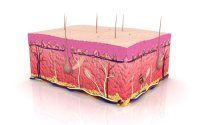 In adult skeletal muscle, where the resident dedicated stem cells («satellite cells») are capable of rapid and highly effective regeneration in response to injury, there is a loss of regenerative potential with age.
In adult skeletal muscle, where the resident dedicated stem cells («satellite cells») are capable of rapid and highly effective regeneration in response to injury, there is a loss of regenerative potential with age.
 Aging refers to the gradual loss of cellular function. And regeneration is the repair of damaged tissue that allows preserving tissue function in an organism. Tissue regeneration is generally mediated by
Aging refers to the gradual loss of cellular function. And regeneration is the repair of damaged tissue that allows preserving tissue function in an organism. Tissue regeneration is generally mediated by  With age, there is a gradual decline in the regenerative properties of most tissues. This decline is linked to a decreased number of stem cells, their dysfunction in
With age, there is a gradual decline in the regenerative properties of most tissues. This decline is linked to a decreased number of stem cells, their dysfunction in  Atherosclerosis provides an example of a chronic disease that involves inflammatory mechanisms. Recruitment of blood leukocytes characterizes the initiation of this disease. Its progression involves many inflammatory mediators, modulated by cells of both innate and adaptive immunity.
Atherosclerosis provides an example of a chronic disease that involves inflammatory mechanisms. Recruitment of blood leukocytes characterizes the initiation of this disease. Its progression involves many inflammatory mediators, modulated by cells of both innate and adaptive immunity.
 Inflammation is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants.
Inflammation is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants.
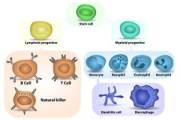 Inflammation can be classified as either acute or chronic. Acute inflammation is the initial response of the body to harmful stimuli. Chronic inflammation is a prolonged inflammation.
Inflammation can be classified as either acute or chronic. Acute inflammation is the initial response of the body to harmful stimuli. Chronic inflammation is a prolonged inflammation.
 An inflammatory process begins as the result of a set of changes. Regulation of immune function declines during aging. Particularly, malfunctioning of niches of hemopoietic cells takes place. As the result, the amount of monocytes and macrophages, which can cause the inflammatory process in the walls of blood vessels or even in brain tissues, increase drastically. Accumulation of DNA damages and disfunctional mitochondria cause excess activation of innate immunity mechanisms. That leads to the formation of
An inflammatory process begins as the result of a set of changes. Regulation of immune function declines during aging. Particularly, malfunctioning of niches of hemopoietic cells takes place. As the result, the amount of monocytes and macrophages, which can cause the inflammatory process in the walls of blood vessels or even in brain tissues, increase drastically. Accumulation of DNA damages and disfunctional mitochondria cause excess activation of innate immunity mechanisms. That leads to the formation of